EU-STANDS4PM harmonised Data Access Agreement (hDAA) for sharing and using controlled access data
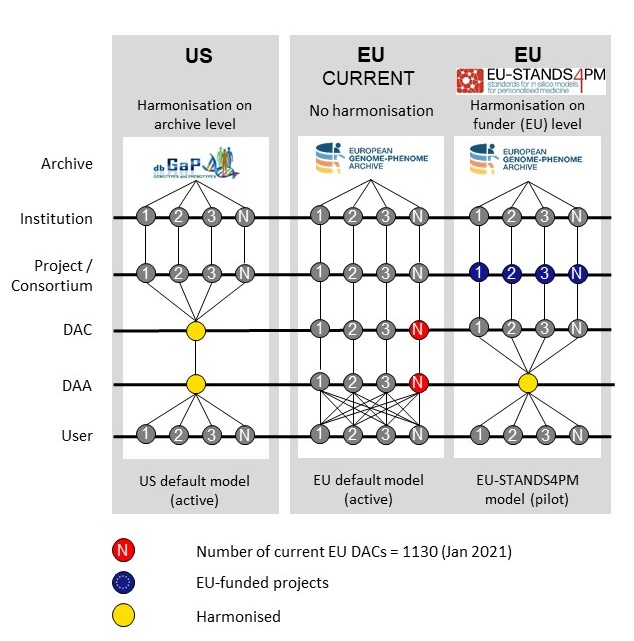
The EU-STANDS4PM new harmonized Data Access Agreement compared to current models for controlled data access.
The replacement of the diverse Data Access Agreements (DAAs) by a single approved harmonized DAA (hDAA) can remove previous barriers to access and sharing of controlled access data (deposited in specific archives). The proposed new hDAA reduces the bureaucracy for both, data access committees (DACs) and prospective data users, and by removing inappropriate clauses present in many DAAs. Hence, the new hDAA for controlled access data is aiming at better governance and flexibility.
A DAA, including the EU-STANDS4PM hDAA, is an agreement between the data provider (controller) and data user (recipient). It is executed via a DAC and defines the details of how the data can be used and stored by the user. The EU-STANDS4PM hDAA has been developed based on the European General Data Protection Regulation (GDPR) as interpreted by the EU-STANDS4PM framework of Ethical, Legal and Social Implications (ELSI) which is available as full and compact version.
The advancement of Precision Medicine is critically dependent on efficient sharing of relevant data. However, the majority of this data is classified as controlled access and over 1000 different Data Access Committees (DACs) and Data Access Agreements (DAAs) from different jurisdictions are currently in use to control the access to such data deposited in the European Genome-Phenome Archive (EGA). To address this issue, EU-STANDS4PM has developed a harmonised Data Access Agreement (hDAA) which is GDPR compliant and improves the sharing of controlled access data for the providers and users of such data.
The current version of the EU-STANDS4PM hDAA is designed to be used in conjunction with new controlled access data being deposited into EGA. It does not replace existing DAAs already linked to deposited data. Implementing the EU-STANDS4PM hDAA is easy. Simply add this link along with a link to the corresponding DAC to any new submission of your controlled access data. A detailed workflow describing the entire process of submitting controlled access data in general is being developed by the IHEC Bioethics Working Group in collaboration with EU-STANDS4PM.
The current version of the EU-STANDS4PM hDAA can be downloaded from EGA and EU-STANDS4PM homepage.
